The Food and Drug Administration on Tuesday issued its first-ever approval of a postpartum depression therapy, offering a new treatment option for the roughly one in nine women who experience this kind of disorder. The question remains, however, of how accessible that option will end up being for patients.
Branded as Zulresso, the therapy supplies women with a compound comparable to allopregnanolone, a steroid which regulates neurotransmission. The body creates more of this steroid during pregnancy, only to have levels return to normal after giving birth. If that reset somehow gets disrupted, the thinking is it can cause postpartum depression to develop.
Three mid- to late-stage clinical trials found postpartum depression patients who received Zulresso experienced significantly greater improvement on a rating scale for depression than those who didn’t. A few patients lost consciousness while on Zulresso, raising concerns about its safety. Still, two FDA advisory committees jointly concluded in November that the therapy’s risk-benefit profile was favorable enough to warrant an approval.
“It’s very difficult to separate from placebo and get statistically significant results,” Walter Dunn, who sits on one of those advisory committees, said of depression drug studies in a March 15 interview with BioPharma Dive. “But here, the first thing that was striking was that all three studies were positive.”
Dunn voted in support of Zulresso’s approval, noting that from an efficacy standpoint the decision is a no-brainer. More challenging will be the conversations around reimbursement and patient access.
Zulresso’s developer, Sage Therapeutics, has set the therapy’s price at $ 34,000 per course of treatment. Chief Medical Officer Steve Kanes told BioPharma Dive in a March 18 interview that conversations with payers have been “in the range of what we think is appropriate for value,” though the company will have a better understanding of appropriate costs after the FDA’s approval and labeling decisions.
Kanes also said that payers seem to understand the need for postpartum depression treatments. Before Zulresso, none had received an FDA nod.
Even so, it’s likely Sage will have to offer substantial rebates on its new product. Investment bank Raymond James, which has since dropped coverage of Sage, estimated in November the net price for Zulresso would be around $ 14,000 per treatment course.
“I’m sure [payers] have their accounts doing the numbers and trying to crunch how to make this most cost-effective,” Dunn said. “Perhaps they restrict it to the most severe patients who may require inpatient hospitalization.”
Zulresso is given intravenously over a 60-hour period. Per its newly minted label, the therapy must be administered in “certified healthcare facilities.” While that doesn’t necessarily mean hospitals, it does rule out patients getting infusions in their homes.
FDA staff have been apprehensive about patients getting Zulresso infusions in their homes because, they argue, family and friends wouldn’t be as well-equipped as a healthcare professional to provide continuous monitoring and the necessary IV bag changes.
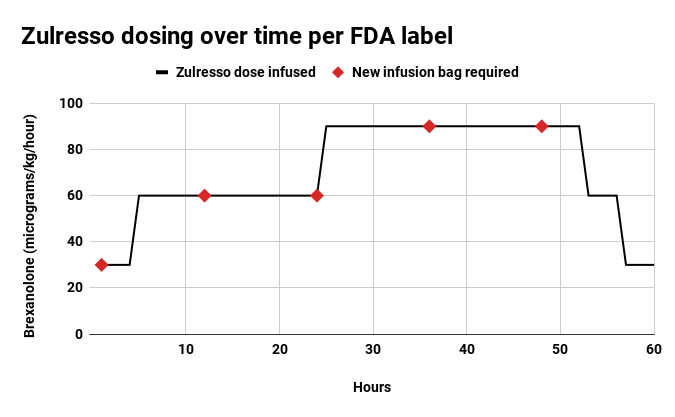
Ned Pagliarulo / BioPharma Dive, data from FDA adcomm briefing documents
Regulators have also worried about women being the primary caregiver for their child while receiving the therapy. The FDA noted in a Tuesday press release that mothers on Zulresso must be accompanied during interactions with their children.
Combined, those requirements may be a deterrent from some patients — particularly single mothers or women who might not have someone else to look after their child over the treatment period.
Sage’s view is that many women would be open and able to giving up two and a half days for a one-time treatment for their postpartum depression. After talking with patients and advocacy groups, the company said it doesn’t see the administration requirements as a barrier to Zulresso’s adoption.
“Like every other severe medical illness, you do what you need to do to feel better,” Kanes of Sage said.
“If we were talking about somebody who had pneumonia or some other condition that can be treated quickly, there wouldn’t be a question about going into the hospital and taking care of it.”
Yet before commercialization can truly commence, Sage needs to get Zulresso scheduled by the Drug Enforcement Administration due to the way it works.
The coming months will test Sage’s theory and rollout strategy. According to recent comments by executives, the company plans to launch Zulresso this June following DEA scheduling, which they expect to occur within 90 days of approval.
Zulresso will carry a boxed warning and will only be available through a restricted program. Healthcare facilities must enroll in the program, and ensure the therapy is given just to patients who are also enrolled. Regulators are asking pharmacies to dispense Zulresso only to those certified, enrolled healthcare facilities, and are mandating wholesalers and distributors to register with the program.
Sage, meanwhile, is already also working on another drug for postpartum depression.
Called SAGE-217, the drug is administered orally and has scored positive results in Phase 3 testing. Sage announced data from a late-stage trial earlier this year showing postpartum depression patients treated with SAGE-217 showed improvement on a depression rating scale that was more than four points higher than those in a placebo group.
Kanes said it’s premature to start speculating about when Sage would file that newer therapy for approval, noting that the company has “some time to go” before it’s done analyzing the recent data readouts.
